In emulsion
polymerization, a dispersion of polymer in aqueous phase (latex) is obtained.
The solids content is usually between 40-65 %w. These polymers are broadly used
in paints, adhesives, rubbers, binders and additives for the paper and textile
industry. The development of reliable mathematical models for emulsion
polymerization requires a high level of understanding of the phenomena taking
place in the reactor. In this article, we offer a brief overview of the basic
steps in batch emulsion polymerization and we discuss the kinetics of the
process. Common monomers employed in this polymerization are styrene, methyl
methacrylate or vinyl acetate.
The monomer
feed is dispersed using water emulsifiers
or surfactants. These are typically
molecules like Sodium Lauryl Sulfate with a non-polar tail and a water soluble
end which stabilize the monomer droplets
in the aqueous phase. The excess of surfactant forms micelles which are swollen with monomer, as it is described in the
following figure:
Polymerization
is started by means of a water soluble initiator (tert-butyl hydroperoxide,
potassium persulfate). Thus radicals are formed in the aqueous phase from the
small amount of monomer dissolved. When the oligoradical chain has grown to a
certain extent, it becomes hydrophobic and enters one of the micelles (the
entry to the monomer droplets is unlikely because of the low total surface area
that these represent). This is called heterogeneous nucleation and as a result
a polymer particle is formed. If the
oligoradical does not enter a micelle, it will continue growing until it
precipitates absorbing emulsifier (homogeneous nucleation). This situation is
described in the following figure:
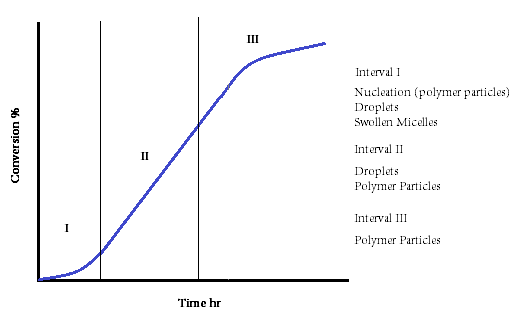
In this
stage there is a coexistence of monomer droplets, monomer swollen micelles and polymer
particles in the reactor. Monomer is consumed inside the polymer particles
according to the free radical polymerization mechanisms. The monomer consumed
is replaced by monomer that diffuses from the droplets. The micelles in the
system decrease because they are converted to polymer particles or they are
destroyed to provide enough surfactant to the growing polymer particles. When
all the micelles disappear (5-10% of monomer conversion), the nucleation
interval has come to an end:
At this
stage, the system consists of polymer particles and monomer droplets. Given
that the mass transfer rate for monomers with higher water solubility than
styrene is higher than the polymerization rate, the monomer concentration in
the polymer particles reaches a maximum and constant value. When solubility is
too low, this stage can be diffusionally limited.
As monomer
diffuses from droplets to particles, these grow until the monomer droplets
disappear. At this point the system is exclusively composed of polymer particles.
The monomer conversion at which this takes place highly depends on the capacity
of the particles to be swollen with monomer (40% for styrene, 15% for vinyl
acetate). In this new interval the monomer concentration is the particles
decreases until polymerization is completed. Finally, polymer particles in a
latex of size varying from 80-300nm are obtained:
These
stages are summarized in the following conversion-time figure:
The rate of
polymerization in the polymer particles is similar to the bulk polymerizations,
where kp is the propagation constant [m3 mol-1
s-1], [M]p is the monomer concentration in the polymer
particles, n is the average
number of radicals per particle, Np the number of polymer particles
and NA the Avogadro’s number:
Since in
emulsion polymerization the radicals are distributed among the particles, the
overall radical concentration is much higher than in bulk polymerization,
resulting in a higher polymerization rate. Radicals have a longer life time leading
to higher molecular weights, and are not terminated until another radical
enters the particle, which is less probable if there are more polymer particles
in the system. Therefore, the polymerization rate and the molecular weight can
be increased simultaneously by increasing the number of particles. This is an
advantageous characteristic of emulsion polymerization that could not be
attained in bulk or solution polymerization.
Carlos
Arnaiz del Pozo













0 comentarios:
Publicar un comentario